Learn
Understanding Polycystic Kidney Disease: Genetics & Reproductive Carrier Screening.
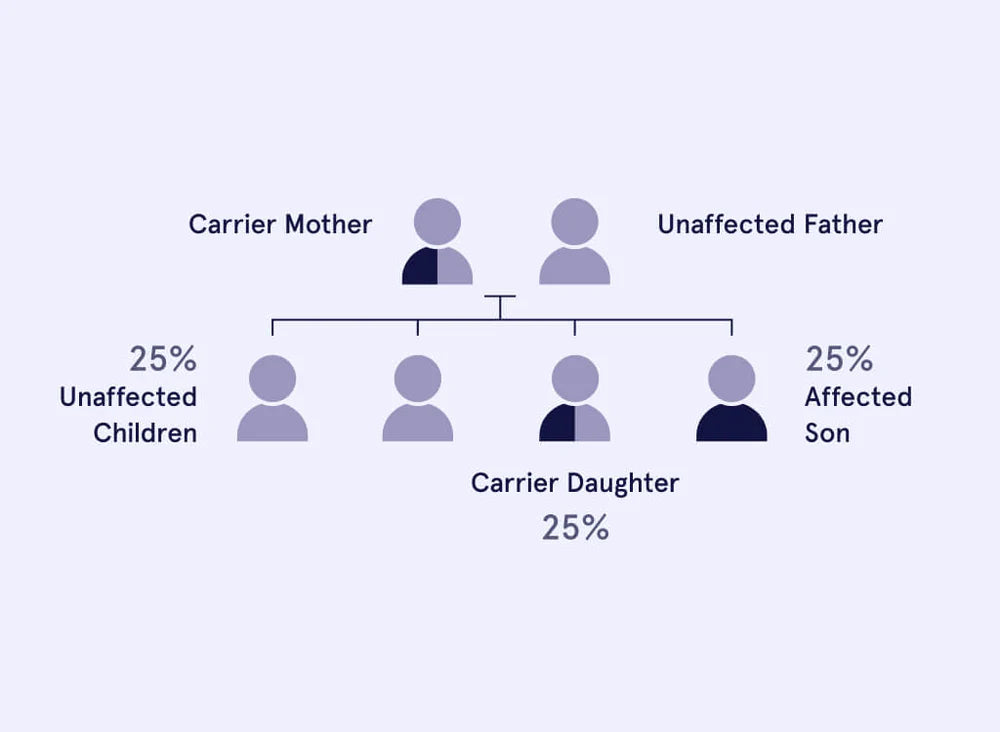
Abbreviation: PKDGene: PKHD1Inheritance pattern: Autosomal recessive (AR) Polycystic Kidney Disease (PKD) is a genetic disorder that primarily affects the kidneys, potentially leading to damage in other organs as well. Understanding this condition, its implications, and available options for reproductive planning is crucial. What is Polycystic Kidney Disease (PKHD1 related)? Polycystic Kidney Disease (PKHD1 related) is characterised by the development of fluid-filled sacs, or cysts, on the kidneys. These cysts hinder the kidneys' ability to effectively filter waste from the blood, resulting in enlarged kidneys and a potential risk of kidney failure. Being a Carrier of Polycystic Kidney Disease Carriers of Polycystic Kidney Disease (PKHD1 related) possess a genetic variation in one of their "PKHD1" genes. Carriers themselves don't experience any symptoms of the condition and are not affected by it. However, if both partners in a couple are carriers, there's a 25% (1 in 4) chance that their offspring will be affected by the disease. Genetics is a Family Affair Genetics plays a role in family health. If you are identified as a carrier: There's a 50% chance of passing the genetic variation to your offspring, making them carriers without showing symptoms. Close family members might also be carriers. Sharing this information can help them understand their risk and options. Carrier Frequency of Polycystic Kidney Disease Ethnic Group Carrier Rate Affected Rate Pan-ethnic 1 in 70 1 in ... Reproductive Planning and Support Carrier screening is essential before pregnancy, offering options like in-vitro fertilisation with pre-implantation genetic diagnosis. During pregnancy, tests can diagnose the genetic status, and ultrasound can detect abnormalities. After pregnancy, clinical examination and genetic testing confirm the diagnosis. Managing Polycystic Kidney Disease While there's no cure, management options are available: Early intervention for breathing difficulties in infants. Nutritious diet for growth-restricted children. Dialysis or kidney transplant for kidney failure. Medication to address symptoms like high blood pressure. Prognosis and Options Affected children often face kidney failure by age 10, with some infants experiencing life-threatening breathing difficulties. Prognosis improves with proper management. If both partners are carriers, options such as leaving it to chance, prenatal diagnosis, IVF with PGD, or other choices can help avoid passing on the disease. Seek Professional Guidance Carrier screening is an essential option for those who want to understand the chance of having a child with Polycystic Kidney Disease. Eugene’s reproductive carrier screening test is designed to let couples and individuals know if they are carriers of PKD, as well as more than 560 other genetic conditions. This information supports people to make informed decisions about their family planning and pregnancy care options. Eugene’s Genetic Counsellors support you before, during and after results and are available to help you make the best decision, based on your values and needs. Explore the latest reproductive carrier screening options fully supported by our genetic counsellors. More information and resources:Polycystic Kidney Disease Australia - https://pkdaustralia.org/Genetics Home Reference - https://ghr.nlm.nih.gov/condition/polycystic-kidney-disease
Understanding Genetic Conditions: Spinal muscular atrophy (SMA)
Genetic conditions are individually rare but when combined are likely to affect 1-2% of babies born every year and while genetic conditions are hugely variable in the way they impact health growth and development, they are rarely curable. Spinal muscular atrophy (SMA) is a devastating genetic disorder that affects the nerve cells in the spinal cord, leading to muscle weakness and atrophy. This condition can range from mild to severe, with the most severe form, known as SMA type 1, appearing in infants before the age of six months. These infants may have difficulty with movements such as sucking and swallowing, and may eventually lose the ability to move or breathe on their own. SMA is inherited in an autosomal recessive manner, meaning that for an individual to be affected they need to inherit two copies of the SMN1 (Survival Motor Neuron) gene with a deleterious variation, one from each parent. Approximately 1 in 40 people are carriers of SMA. Carriers are healthy and have no way of knowing this unless reproductive carrier screening is performed as part of pre pregnancy planning of early pregnancy care. When a couple are both found to be carriers of SMA, there is a 25% (1 in 4) chance with each pregnancy of having a child with SMA. Carrier screening is recommended by RANZCOG and RACGP (the governing bodies for Obstetricians and GPs in Australia and New Zealand). For couples who find out that they have a high chance of having a child with SMA there are many options they can consider to reduce the chances of having an affected child. These can include, but are not limited to prenatal diagnosis of a naturally conceived pregnancy or IVF with Preimplantation Genetic Testing (PGT). For couples who know of their risk in advance, medicare support is available for both prenatal diagnosis and IVF. Your doctor is likely to recommend speaking to a genetic counsellor about your options. Overall, SMA is a serious genetic disorder that can have a significant impact on individuals and their families. While the management and treatment for SMA has come a long way, it is still considered to be a life limiting condition Even though there is no cure, there are treatment options available to help manage the symptoms of the condition and improve the quality of life for those living with SMA. One of these options is drug treatment called Nusinersen (marketed as Spinraza). This drug works by increasing the amount of the SMN protein, which is essential for the survival of motor neurons. It has been shown to improve muscle function and mobility in individuals with SMA. Another potential treatment option for SMA is gene therapy. In recent clinical trials, gene therapy has shown promise in improving muscle function and supporting life in individuals with SMA. However, more research is needed to fully understand the effectiveness and safety of this approach. Carrier screening is an essential option for those who want to understand the chance of having a child with SMA. Eugene’s reproductive carrier screening test is designed to let couples and individuals know if they are carriers of SMA, as well as more than 560 other genetic conditions. This information supports people to make informed decisions about their family planning and pregnancy care options. Eugene’s genetic counsellors support you before, during and after results, and can help you make the best decision based on your values and needs. Explore the latest reproductive carrier screening options fully supported by our genetic counsellors.
Navigating Genetic Disparities in Healthcare: Addressing Racial Differences in Disease Risk
As at-home genetic testing grows with the upcoming Medicare rebate for carrier screening, patients will bring their results to physicians for reaction and response. Physicians will need to be proactively prepared. Providers need to educate themselves about the critical differences that exist in their patient populations. Health disparities, while driven by many social factors, result from some clinicians needing to apply known nuances in the care of special populations. Today, we revisit a study published on The Conversation by MD Greg L Hall. Geneticists, meanwhile, are also getting more tailored information about disease risk and prevalence as genetic testing in medical research centers continues. Physicians accept that cystic fibrosis, for example, is much more common in people with Northern European ancestry and that sickle cell disease occurs dramatically more often in people with African origins. These commonly accepted racial and ethnic differences in disease prevalence are just the tip of the iceberg when looking at clinical differences that vary based on genetics. But there’s a problem, a recent study from the National Institutes of Health found. Many physicians and other providers are uncomfortable discussing race with their patients, and also reticent to connect race or ethnicity to genetics and clinical decision-making, the study suggested. Overall, physician focus groups “asserted that genetics has a limited role in explaining racial differences in health,” the authors added. As a primary care physician who teaches urban health to medical students and as a state minority health commissioner who advocates for health equity, I see this as a problem that health care systems, and their providers, need to address. The state of the science Commercial DNA tests, such as those provided by 23andMe, not only give people their racial and ethnic lineage but also can provide a weighted risk for diabetes, stomach ulcers, cancer and many other diseases. In April, the FDA granted approval to 23andMe to sell reports to consumers that tell them whether they may be at heightened risk. These companies already have the data that describe the risks for health problems based on the percentage of their ancestry composition. Those differences have been published and known in academic circles for many years. With the widespread availability of DNA tests, patients will now know their increased individual risks. For example, Ashkenazi Jews, a specific Jewish ethnic population originating from Central and Eastern Europe, are known for having a disproportionate occurrence of a number of diseases, including Tay-Sachs disease, amyloidosis, breast cancer, colon cancer and many more. The BRCA1/2 gene mutation greatly increases the propensity for breast and colon cancer and occurs in 1 in 40 people of Ashkenazi Jewish heritage, whereas 1 in 800 Americans in general carry that mutation. This 20-fold increased risk should prompt more aggressive screening for the gene, and more frequent and earlier mammography and colonoscopies in Ashkenazi Jews compared to the general population. Relatively higher rates of these cancers occur in certain populations, such as Ashkenazi Jews, and demonstrates the need for more nuanced care based on data that is already available. But this information is too infrequently accessed by providers. Genetics knowledge growing fast African-Americans are another group with higher rates of certain genetically driven diseases. African-American men have an increased occurrence of prostate cancer, kidney failure, stroke and other health problems. Prostate cancer in African-American men, for example, grows faster and metastasizes four times as often than in European-Americans. But despite this increased risk for prostate cancer, doctors’ use of the PSA (prostate specific antigen), a test that works well with identifying prostate cancer in African-Americans, has steadily decreased due to recommendations aimed at majority patients who come from European-related heritage. In European-Americans, prostate cancer can be more indolent and occurs at a lower rate than African-Americans. Also, certain types of blood pressure medications – ACE inhibitors, for example – lead to worse outcomes in African-Americans when used singularly as first-line therapy for high blood pressure, yet these medications work very well in Americans of European decent, a large study of hypertension therapy found. A follow-up study that looked at subsequent clinical practices – which was done in response to changed recommendations based on race – showed nearly a third of African-American hypertensive patients continued to be prescribed medications that cause worse outcomes. African-Americans also have a four-fold increased risk for renal disease leading to dialysis. Geneticists suspect that they have identified the gene that drives this difference yet most clinicians do not have the resources to test for this gene and identify the 30 percent of African-Americans that carry it. And a gene that greatly increases the risk for Alzheimer’s disease, APOE-4, has also been identified and occurs disproportionately higher in European-Americans yet is almost nonexistent in African-Americans and is inconsistent in Hispanic-Americans. Great controversy exists surrounding the testing for this gene, given the devastating impact it could have on a patient or family. (Hispanic and African-Americans still have a very significant risk for Alzheimer’s disease, but it is not driven by this gene). Genetically different responses to medications Patient response to medications vary according to the presence or absence of genetic variants, which can impact the dose and the effect of many pharmaceuticals. Some of these differences can be anticipated based on race or ethnicity. For example, Warfarin is a commonly used medication in the treatment of a number of cardiovascular disorders including atrial fibrillation, deep vein thrombosis and heart valve replacement. It shows wide variations in dosing, with Americans of Asian descent requiring less medication and African-Americans requiring more to achieve equal effects. European-Americans have a variant gene that make having a major bleed on Warfarin much higher. A popular cholesterol-lowering medication, Rosuvastatin, better known as trade name Crestor, is twice as powerful in patients of Asian descent, and their manufacturing label indicates starting at a much lower dose in this population. In fact, the highest manufactured pill dose of Crestor is “contraindicated in Asian patients.” Patient-centered care is the key Because of the “patient-centered” movement in hospitals, clinics and insurance plans, providers are now feeling increased pressure to improve the quality of care provided to individual patients. Many outcomes and patient cost of care are now tracked by providers. And countless well-designed studies have validated verified differences in the clinical care of a number of pervasive diseases based on ancestry. Providers need to educate themselves about the important differences that exist in their patient populations. Health disparities, while driven by a number of social factors, are also the result of some clinicians not applying known nuances in the care of special populations. As home genetic testing grows, patients will be bringing their results to physicians for reaction and response. Physicians will need to be proactively prepared. This article was originally published on The Conversation. Read the original article. Author: Greg Hall, Case Western Reserve University Gregory L. Hall, MD, is a primary care physician practicing in Cleveland, Ohio for over 20 years. A native Clevelander, he attended Williams College and majored in psychology while taking pre-med coursework. In 2010, he was appointed to the Cuyahoga County Board of Health which oversees Ohio’s largest county’s broad range of quality driven public health programs and services.
How to Understand Your Genetic Cancer Risk
At Eugene, our mission is to make essential health insights accessible. Our tests are designed for people who are eager to understand their genetic makeup and make informed decisions about their health, particularly when it comes to the risk of specific cancers. We understand that the thought of developing cancer can be scary, and it's natural to want to take proactive measures to safeguard your health and that of your family. We offer support and genetic guidance every step of the way. Let's jump right in! Step 1: Order Your Proactive Cancer Risk Test Online: Ordering your cancer risk test is quick and easy with Eugene. Simply order the cancer risk test on our website. We offer free, prompt delivery to your doorstep anywhere in Australia. Step 2: Provide Your Health Background: Your genetic makeup is unique, and so is your health story. When you order your test kit, you'll have the opportunity to share your personal health history, family history and health goals. You can also provide your doctor's details so we can collaborate to get a holistic understanding of your health journey. Step 3: Give Your Saliva Sample: Providing a saliva sample for genetic testing is simple, non-invasive, and can be done in the comfort of your own home. The test kit we provide includes everything you need to collect a sample and return it to us using the provided pre-paid mailer. Step 4: Access Health Insights with Expert Genetic Counselling: After our accredited laboratory analyses your genetic information, we'll send you a comprehensive report with insights into your cancer risk. We understand that understanding these complex concepts may feel overwhelming, which is why we provide ongoing support through our certified genetic counsellors. You'll have the opportunity to schedule a video consultation with them to discuss your results and answer any questions you may have. Conclusion Deciding to proactively manage your genetic cancer risk is a significant step towards being proactive about your health. And here at Eugene, we're committed to offering comprehensive, accessible, and easy-to-use support to guide you through every step of this journey. No matter your family health history or personal goals, we're here to empower you with knowledge and confidence. So, take action, order your test kit today and start managing your genetic cancer risk.
How to get a genetics test
Unlocking health insights has never been easier. At Eugene, we provide simple and accessible genetic testing options for people looking to explore their genetic makeup and for people thinking about having a baby. In this guide, we'll walk you through how to get a genetic test in the easiest way possible. Step 1: Order Your Test Online Your journey toward personalised genetic insights is just a click away. We’ve removed the barrier that referrals and forms can present. Simply choose the genetics test that is right for you – whether it's family planning or understanding potential health risks. Once you've decided, we'll take care of the rest, delivering your test kit to your door, with free delivery. This is healthcare designed around your life, giving you control over your journey. Step 2: Share Your Health Story At Eugene, we understand that everyone has their own unique story. After you’ve ordered your genetics test, tell us a bit about your health history and goals. This helps us customise your results to your specific situation, so the insights you get are relevant and actionable. Sharing your doctor's details also lets us collaborate to provide precise and helpful information. Your path is unique – and our approach reflects that. Step 3: Collect your sample Collecting your genetics sample with Eugene is a breeze. Simply spit in a tube, pop it into the prepaid return package we provide – and you're finished. No needles, no discomfort, no worries. A few moments of providing saliva is all it requires. This straightforward process allows you to better understand your genetics from the comfort of your own home. Step 4: Discuss Your Results with a Genetic Expert Once we receive your sample, our internationally accredited lab starts analysing your genetic information. These test results are typically ready within 3-6 weeks. Book a video call with one of our certified genetic counsellors to walk through your report. This session is all about making things clear and holding space for you to ask questions, so you know what to do with your results. We're here for you every step of the way of your genetic health journey. Conclusion: Genetic Insights to empower you Getting a genetics test with Eugene is a pathway to empowerment, understanding yourself, and personalised healthcare. With a commitment to simplicity and empathy, we've redefined how genetics testing can fit seamlessly into your life. You can take control of your health narrative today and unlock the insights that could shape your future choices. Our team is here to help you navigate this journey with confidence.
What is Cystic Fibrosis?
Cystic fibrosis (CF) is a genetic condition that affects many organs in the body: especially the lungs, pancreas and sweat glands. People affected by CF produce abnormally thick, sticky mucus, particularly in the lungs and digestive system. Intelligence is not affected in people with CF. While it is normal to have mucus lining the organs of the respiratory, digestive, and reproductive systems, in people with CF this mucus is thick and sticky which can trap infections in the lungs leading to progressive lung damage. Digestion is affected as the body cannot extract nutrients from the normal diet, this can be improved by taking enzyme supplements however, commonly people with CF struggle with maintaining healthy weight despite a good appetite and balanced diet. FInally, the sweat glands also secrete sweat that is very high in salt, thereby depleting the body of this important substance. The severity of symptoms varies from person to person, even among individuals in the same family and/or with the same mutations. Males with CF may also appear as infertile. This is because most men with CF are missing the tubes that transport sperm from the testicles out through the penis - this is called congenital absence of the vas deferens (CAVD). Men with CF still produce normal mature sperm and thus infertility can be overcome using IVF. How frequently is it carried by individuals (Carrier Frequency)? Ethnicity influences the chances of carrying the Cystic Fibrosis mutation. Cystic fibrosis affects most commonly people who are of Northern European or UK descent, fairly frequently people whose ancestry is Southern European and Middle Eastern populations, but is rare where the ancestry is Asian. When and how is it diagnosed? Before a child is born Pre-pregnancy screening can identify couples at risk of having a child affected with CF. It is important to know that CF is not part of standard first trimester genetic tests and may sometimes be found by chance during routine second trimester ultrasounds. If a pregnancy is known to be at risk a test may be requested and/or offered to diagnose the genetic status of the pregnancy. This can be done as early as the 11th week of pregnancy. At birth: Most cases of CF are diagnosed following Newborn screening which is a heel-prick blood test offered to all newborns internationally and aims to identify babies that have an abnormal level of certain chemicals in their blood. Testing of people thought to be at risk may include a sweat-test to measure the concentration of salt in the sweat, and then by genetic testing of the CFTR gene. More than 1000 spelling mistakes (mutations) have been shown to affect the function of the CFTR gene and thus genetic testing is the ultimate tool in diagnosis. In general, individuals with two classic mutations are more likely to have a severe form of the disease including problems with the pancreas, while individuals with one classic and one non-classic or individuals with two non-classic mutations are more likely to have a milder form of the condition and may avoid problems with the pancreas. It is important however to remember that not all people with the same mutation will have the same severity or prognosis. Most people with CF experience breathing problems and frequent lung infections that lead to permanent lung damage such as scarring (fibrosis) and sac-like growths (cysts). The pancreas, an organ that produces insulin and digestive enzymes, is often affected by CF. The sticky mucus caused by CF can block ducts which ferry enzymes from the pancreas to the rest of the body, resulting in problems such as diarrhea, malnutrition, and poor growth. Infertility, particularly in men, and delayed puberty are also common among people with cystic fibrosis. How can it be treated? There is no treatment that addresses the cause of CF, but there are many options to treat the symptoms it produces. Daily physiotherapy in combination with prescription medication can help clear mucus from the lungs, improving lung function and reducing the risk of infection. As respiratory infections occur, physicians typically prescribe antibiotics. SOmetimes people will require hospital admissions for health support. Physicians will also monitor the digestive system to ensure that the person is getting proper nutrition. Enzymes or vitamin supplements may be prescribed. Both the respiratory and digestive systems of a person with CF must be monitored regularly by his or her medical team. Surgery may be needed to correct certain problems caused by CF. Lung transplants are an option for some people. More recently genetic therapies have shown to improve the quality of life of people with CF. What is the prognosis for a person with Cystic Fibrosis? There is no cure for CF. However, thanks to improved treatments and a better understanding of the condition, the average life expectancy for people with CF who live to adulthood is 35 years. Children born with CF today who receive early treatment may live even longer. Resources Cystic Fibrosis Foundation (USA) Cystic Fibrosis Australia
From 3 Genes to 780: The Evolution of Reproductive Carrier Testing
Reproductive carrier screening is a crucial aspect of family planning, allowing couples to assess their risk of passing on genetic conditions to their children. While traditional carrier screenings for a limited number of conditions have been available at your doctor’s office, such as the three gene test - there is a growing consensus among rare disease advocacy groups and medical organisations, including the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), that larger panels of genetic tests offer numerous advantages. Let’s delve into the significance of expanded and comprehensive carrier screenings and why they are increasingly recommended for all couples, no matter where you are from. Understanding Expanded and Comprehensive Carrier Screenings Traditional carrier screenings typically involve testing for a small number of genetic conditions that are common in certain ethnic backgrounds. For instance, a 3-gene test may be more suitable for White Northern European individuals with a higher risk of carrying specific genetic mutations like cystic fibrosis, spinal muscular atrophy, and fragile X syndrome. On the other hand, expanded and comprehensive carrier screenings offer a more inclusive approach, analysing a broader range of genetic conditions. These screenings may encompass hundreds to over a thousand conditions, providing individuals and couples with a more comprehensive understanding of their genetic risk factors. Comprehensive panels, which can include to over 780 conditions, are particularly advantageous because they consider genetic variations that are relevant across diverse ethnic backgrounds. Importance of Comprehensive Panels Inclusivity Across All Backgrounds Genetic diversity is prevalent in human populations, and different ethnic groups may carry distinct genetic mutations that can lead to various inherited conditions. Comprehensive panels ensure that individuals of all backgrounds receive appropriate and accurate screening, eliminating potential bias towards specific ethnicities. Early Detection and Informed Decision-Making Comprehensive carrier screenings not only detect more genetic conditions but also allow for earlier identification of potential risks. Armed with this knowledge, individuals can make more informed decisions about their family planning options, including assisted reproductive technologies or prenatal testing, leading to better health outcomes for future generations. Identifying Rare and Uncommon Variants While traditional screenings focus on prevalent genetic mutations, comprehensive panels also identify rare or less common variants that might not be covered by narrower tests. Uncovering these lesser-known mutations can prevent passing on the lesser-known genetic conditions within families, they may be lesser known but still serious or life-threatening. Potential Cost Savings Despite their initial higher cost, comprehensive panels can be more cost-effective in the long run. Instead of repeating multiple tests for different genetic conditions, a single comprehensive screening can cover a wide range of potential risks, ultimately reducing overall healthcare expenses. Advancements in Genetic Technology Recognising the advantages of comprehensive carrier screenings, RANZCOG and other medical organisations have recommended a broader approach to genetic testing. This progressive stance aligns with the global movement towards more inclusive and personalised healthcare, considering the diverse genetic makeup of our populations. Furthermore, advancements in genetic technology have made it possible to analyse a large number of genes simultaneously, making comprehensive panels more accessible and affordable. The reduction in costs and increased availability of these tests ensure that all individuals, regardless of their ethnic background, can benefit from the knowledge they provide. In conclusion, reproductive carrier screening plays a vital role in family planning and ensuring the health of future generations. As medical knowledge and technology continue to advance, the shift towards expanded and comprehensive carrier screenings has become increasingly important. RANZCOG's recommendation for larger panels reflects the commitment to inclusivity and personalised healthcare for all individuals, regardless of their ethnic background. By opting for comprehensive screenings, we empower couples to make informed decisions and promote the well-being of their families in the most holistic way possible. At Eugene, we know it’s important to offer an inclusive range of reproductive carrier screening, available with or without your GP’s referral.
A Quick Guide to Genetic Testing before and during Pregnancy
For expecting parents, the health and well-being of the baby is everything. Genetic testing during pregnancy is a valuable tool for identifying potential genetic disorders or abnormalities in your baby’s DNA that could impact their health both during development and throughout their life. Knowledge is power, and the more informed you are about genetic testing, the better equipped you will be to make important decisions that impact your baby's health. In this quick guide, we’ll take a closer look at the types of genetic tests available, the factors that guide selection of the right test, the emotional and ethical considerations involved in genetic testing, and the role of the healthcare provider in the process. Types of Genetic Tests during pregnancy: Carrier Screening: Carrier screening is usually conducted before conception or early in pregnancy and checks if prospective parents are carrying any genetic mutations that could be passed on to their children. Carrier screening tests are typically recommended based on ethnic background or family history. Non-Invasive Prenatal Testing (NIPT): NIPT is a screening test done during pregnancy that checks for common chromosomal conditions, including Down syndrome, trisomy 13 and 18. This test is non-invasive and carries no risk of miscarriage. Prenatal Diagnostic testing: Prenatal diagnostic tests are usually conducted after the 10th week of pregnancy and can detect genetic disorders that the baby might have inherited from both parents. These tests are more invasive and carry a small risk of miscarriage. Factors That Guide Selection of the Right Test: Several factors guide the selection of the right test, including personal medical history, family history, and ethnic background. Depending on these factors, healthcare providers evaluate which test is most appropriate for parents. For example, if there is a history of genetic disorders in the family, prenatal diagnostic testing might be recommended. Similarly, carrier screening is often recommended for individuals from certain ethnic groups, such as those of African or Mediterranean descent, who have an increased chance of carrying specific genetic mutations. Emotional and Ethical Considerations: Genetic testing can have emotional and ethical implications for parents. A positive test result can cause significant anxiety and distress, and discussing these outcomes with a healthcare provider or counselor during the decision-making process can help parents prepare emotionally. Additionally, ethical considerations arise when using genetic information to make decisions about the baby's health. Parents should discuss these considerations with their healthcare provider or counselor as part of the decision-making process. The Role of the Healthcare Provider: Genetic testing can be complicated and stressful. The healthcare provider’s role is to guide parents through the decision-making process, manage the emotional implications of positive test results, and offer support and advice throughout the process. Healthcare providers can help parents understand the results of the tests and guide them through the available options. You can also consider speaking with a genetic counsellor to understand your genetic testing options in pregnancy. Summing up: In conclusion, genetic testing is an important decision for parents that impacts their baby's health. Understanding the types of tests, the factors that guide selection of the appropriate test, and the emotional and ethical considerations involved in genetic testing, as well as seeking guidance from healthcare providers, can help you make informed decisions. Starting the conversation about genetic testing early in pregnancy gives you ample time to make the right decision and ensure that your baby receives the best possible care.