In order to be able to ‘select’ an embryo – we need to first understand IVF, as this is the process that gives rise to embryos in a laboratory setting.
What is IVF?
The process of IVF involves using hormones to stimulate a woman’s ovaries to produce a number of eggs in one menstrual cycle. While a natural cycle would only typically produce one mature egg (that is able to be fertilised by sperm), IVF aims to increase this number. The number of eggs collected from an IVF cycle varies from 1-20ish. There are a number of variables that influence this number including the woman’s age, ovarian reserve, response to treatment and luck (even though this is not officially science!)
It is important to know that the number of eggs doesn’t always equate the the same number of embryos. I like to think of IVF like a hurdles race – the stumbling block being that once you miss a hurdle you are out of the race.
Turning embryos into eggs
Eggs are collected from a woman ovaries by a specialist IVF doctor. These eggs are then ‘introduced’ to a sperm sample from the proposed father or donor. There are 2 Fertilisation techniques.
- A technique called ICSI (intracytoplasmic sperm injection) involves injecting a single viable sperm into each mature egg.
- Or by exposing the eggs to a large sperm sample (from the proposed father or donor), in crude terms letting the strongest sperm fertilise the egg.
A large majority of IVF, and all PGD is done using ICSI and in general, fertilisation rates are higher using this technique. However, with all invasive techniques come minor increases in risk of adverse outcome. So doctors are always weighing up the risks with the benefits.
How embryos are selected?
In the case of embryo selection there are 2 main ways an embryologist (this is a scientist that is specialised in creating and supporting the growth of embryos) can ‘select’ an embryo.
Visual assessment
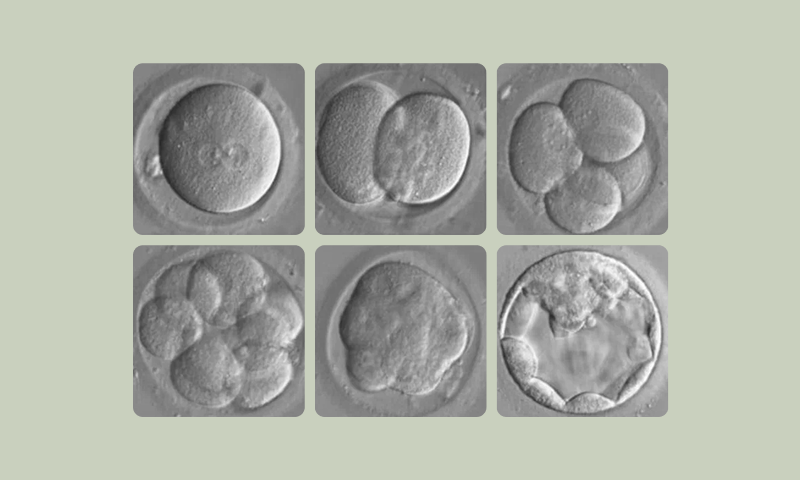
Visually – new technologies called an Embryoscope gives real-time visualisation and tracking of an embryos growth in the laboratory. Embryos are usually grown 5 days until they contain more than 100 cells – this growth stage is called blastocyst – embryos are graded and sorted for potential transfer back to a woman uterus. Typically only 1 embryo will be put back at a time to reduce the risk of multiple pregnancies. Any additional are frozen using technique called vitrification. Embryos are stable in this state and a generally stored for future us by the couple.
Genetic analysis
Preimplantation Genetic Screening (PGS) is used to assess the chromosome make-up of the embryo. We know that most early miscarriages are due to chromosome errors. An example of this is down syndrome – where an embryo inherits 3 copies of chromosome 21, instead of the regular 2 copies. Any chromosome imbalances in an embryo increase the risk of miscarriage and will lead to pregnancy complications and abnormal birth outcomes.
Preimplantation Genetic Diagnosis (PGD) is used to screen embryos for specific single gene faults that are known to be carried by the parent or parents having IVF treatment. Scientists create a unique DNA fingerprint of the specific faulty gene in the family. By comparing the DNA fingerprint of the biopsied embryos to the DNA fingerprint of the family members it is possible to determine which embryos have inherited the disease and which are free of the disorder. Unaffected embryos will then be available for IVF treatment in future cycles with a chance that it will produce a successful pregnancy and a healthy baby.